Menu


Logistics
- Bi-Coastal coverage via our unified San Diego headquarters.
- Recall logistics support services
- Exchange pools, safety & seed stocks
- Warehouse temperature monitoring
- International shipping
Assembly
- Certified ISO 13485:2016
- FDA Registered
- Contract Assembly
- Complex kitting and labeling
- HIPAA Compliant
Technical
- Class 1 & 2 medical devices + pharma
- API, EDI, CSV,FTP integrations
- E-Commerce B2B, B2C, custom portals
- 24/7/365 custom reporting & dashboards
- Serial number and lot tracking
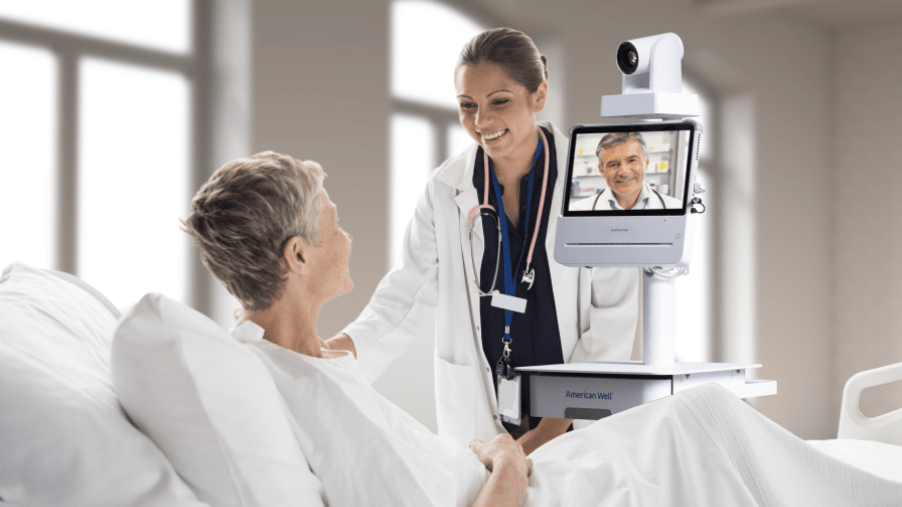
Medical Devices
Contract assembly, medical 3PL, refurbishments & additional supporting services. FDA registered, HIPAA compliant and Certified ISO 13485:2016.

Third-Party Logistics
B2B/B2C, warehousing transportation, complex kitting, labeling, printing and much more. DOT & IATA compliant
Logistics & Assembly Needs?
Comprehensive Services Start Here
Testimonials

★★★★★
Read More
The Mendtronix team is the best in the business!!! I would advise anyone to try out the Mendtronix service company!”
★★★★★
Read More
"As a global company, we needed a full-service solution designed for scalability and continuous optimization which Mendtronix has successfully delivered."

★★★★★
Read More
“At the terminus of Nalu’s supply chain, MTI is a valued partner responsible for warehousing, kitting and labeling of a diverse SKU matrix in support of our FDA regulated operations.”

★★★★★
Read More
Mendtronix has been an amazing partner dedicated to our success, while allowing Flume to serve our customers with the highest level of quality and technical capability.

★★★★★
Read More
MTI works diligently to make things happen, our business required specific licenses and they moved quickly to meet that obligation!

★★★★★
Read More
Mendtronix provides various services for our complex product at the absolute highest level. Their service is always excellent, timely and professional, and we would highly recommend them to any company.

★★★★★
Read More
The MTI team are a pleasure to work with. They are very knowledgeable in 3PL warehousing and the shipping and handling of our products. I would recommend them to any company looking for a quality 3PL.

★★★★★
Read More
“We have found Mendtronix to be an excellent company to work with, very reliable and responsive to our wide range of needs. We would certainly recommend their services.”
Previous
Next
connect with a pro
Fill out the form below, and one of our team members will reach out to you asap.
"*" indicates required fields
